

Ventilated film cans
Ventilated film cans
Mick Newnham investigates film can design and the use of ventilation to remove the catalysing acids that accelerate film decomposition.
One of the major issues in film preservation is the formation of free acids as a byproduct of the decomposition reaction of the cellulose ester polymers used as film base. This process, known as deacetylation, occurs when an acetyl group detaches from the cellulose chain and combines with water to form acetic acid.
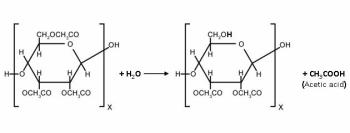
Once a certain level of free acid has formed, the reaction will become catalysed by the acid. Since the catalyst is produced by the decomposition reaction the process is known as an autocatalytic reaction and the level of free acid where the reaction becomes autocatalytic is known as the onset or autocatalytic point.
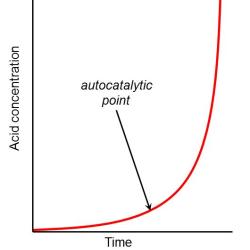
At acid levels beyond the autocatalytic point the decomposition reaction progresses more rapidly at a given temperature than would occur under conditions of low acid concentration at the same temperature.
It is beyond this autocatalytic point that archivists consider a film to have Vinegar Syndrome. And it is Vinegar Syndrome that is proving to be the greatest threat to the long-term survival of film collections.
As a result of the decomposition reaction the film base will shrink markedly and distort. The lower pH causes accelerated fading of colour dyes. The final stage is the complete destruction of the film as the cellulose chain breaks down, leaving a greyish powder.
Once the decomposition reaction has reached the autocatalytic point, the reaction cannot be stopped.

The most commonly used and effective method of controlling the rate of the decomposition reaction, and thus the amount of acid in the film, is by low temperature and low relative humidity storage. Even beyond the autocatalytic point this will have an effect in reducing the rate of reaction.
Establishing suitable vaults with effective insulation and moisture membrane sealing, air handling plant and the associated ongoing running costs are very expensive and archives are typically poorly funded.
Since the decomposition reaction is autocatalysed by the acid byproduct, several methods have been proposed to reduce or remove the acid decomposition byproducts:
- Neutralisation treatments using alkali baths to neutralise free acids in the film system (emulsion and base) – this approach to reducing the rate of reaction is to remove or control the level of acid within the film;
- Kodak/FPC Molecular Sieve-Acid Scavenger – uses zeolites as a passive sorbent to capture acids in the film can micro-environment;
- 'Dry Treatment’, developed by the Vietnam Film Institute (VFI) – film is loosely unwound and placed in a high air exchange environment for 24 hours; during this time the decomposition acids diffuse from the film.
All these methods require a relatively high level of resources to carry out. Apart from the resource implications there are other drawbacks that limit the potential success of their application.
The neutralisation bath involves a very high degree of risk to the film materials. The acid byproduct of the decomposition reaction increases the solubility of the emulsion gelatin to such an extent that it becomes feasible for the emulsion to dissolve in the bath during treatment.
The kinetic energy of the vapours is very low and there is little energy to move the acid vapours into the sorbent; this requires a flow (positive pressure). This significantly reduces the sorption potential of molecular sieves inside a film can where there is no air movement.
The Dry Treatment in its current form as practised in the VFI, or even with a higher degree of mechanisation, has a degree of risk of physical damage and the time-out-of-storage considerations.
Both the Molecular Sieve and Dry Treatment approaches use diffusion to lower the level of acid within the film. By diffusion the acid is released from the surface of the film as a vapour. The vapour will flow from an area of high concentration to one of lower concentration.
The diffusion of gases, in this case acetic acid vapour, is driven by the kinetic energy of the gases. Fick’s First Law of Gas Diffusion describes diffusion as a response to a concentration gradient expressed as the change in concentration (C) due to change in location (x).
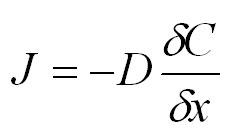
Where:
| J | gas flow (g cm-2s-1), perpendicular to the planes of equal concentration and pointing towards the regions of lower concentration |
| D | the diffusion coefficient for that gas in air |
This is more easily seen on a graph.
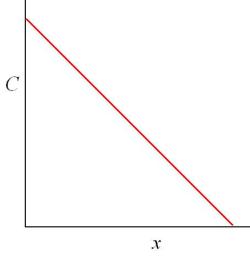
For maximum efficiency in removing the decomposition acids by diffusion from the film the surrounding environment must have a zero concentration. As the concentrations inside the film and the outside environment approach equilibrium the emission rate reduces until at equilibrium the rate is zero. However given that the decomposition reaction is continuing there will always be a slightly higher concentration in the film. A steady state concentration can be reached where the total mass of the gas leaving the material will equal the total mass of the gas leaving the film can.
The linear relationship between emission rate (ER) and concentration can be expressed:
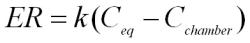
Where:
| k | the pollutant transfer coefficient |
| Ceq | the equilibrium concentration  when ventilation = zero when ventilation = zero |
| Cchamber | the concentration in the chamber (eg film can) at time = zero |
A steady state concentration can be reached where the total mass of the gas leaving the material will equal the total mass of the gas leaving the film can.
This is described by the Hoetjer Equation.
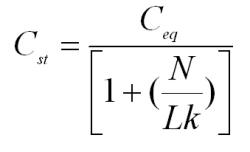
Where:
| Cst | the steady state concentration |
| N | the ratio of airflow over the chamber volume |
| L | the loading or ratio of the object surface area over the chamber volume |
In a film can a steady state concentration will be reached when the concentration inside the film is equivalent to the concentration inside the film can. Any air movement through the film can will lower the steady state concentration. If the flow of air through the film can is sufficient to maintain the concentration at zero then the rate of diffusion from the film will be maximised.
The wind tension under which motion picture films are stored has been shown to be critical in the rate of decomposition as measured by acid content. The effective surface area of the film is difficult to calculate. The difference in the pack diameter of 700’ of film between a tight wind under a tension of 350 g and a preservation wind with 200 g of tension is less than 1%. However the apparent effectiveness of preservation winds in reducing the acid content of films is far greater than the slight increase in surface area based on pack diameter would indicate.
To investigate whether positively ventilating a film can would have any effect on the free acid level of a decomposing film a series of experiments were undertaken.
Experiment 1
The first experiment was looking at any change in free acid concentration by comparing test films with a known free acid content at the start. The test films were analysed for free acid content by the method described by Peter Adelstein and his colleagues in the May 1995 SMPTE Motion Imaging Journal.
Each film was then divided into two equal lengths, each having the same acid content. The films were preservation wound (approximately 200 g tension) on a 75 mm core. One film from each pair was stored in a non-vented can and the other film in a vented can.
The experiment was conducted in pairs of cans, in each pair:
- test can one (1) was non-vented and used as a control;
- test can two (2) was vented by 10 mm diameter holes cut into the sides of the can with aligned holes cut in the sides of the lid.
Each can had a neoprene grommet let into the side of the can. The detector tube makes a tight seal against the grommet ensuring that no air entered around the grommet during sampling. The grommet was covered by polyester tape, except when readings were being taken.
Each can had a 2000 g weight placed on the lid to simulate storage condition in a stack.
The films were stored at 20oC /~50%RH in a constant airflow of 0.3 m/second. A standard airspeed of 0.3 m/sec (~1 km/h) was chosen as a typical air movement in a vault.
The concentration of acetic acid in the micro-environment within the can was measured at regular intervals through the grommet in the side of each can using a Kitegawa detector tube: 216S – Acetic Acid 1 – 50 ppm (parts per million).
Experiment 1 Results
| Can type | Concentration (ppm) |
|---|---|
| Non-vented | >100* |
| Vented | 4 |
*beyond the resolution of the test method
After 12 months the films were again analysed for their free acid content.
The head sample readings on vented cans showed approximately a 35% lower acid content than the non-vented. As the samples were taken further in the reels the difference in acid content decreased until the centre of the reels showed an imperceptible difference between vented and non-vented cans.
Experiment 1 Conclusions
The lower steady state concentration surrounding the film predictably gave a lower free acid concentration in the outer layers of the film.
It appears that the limited ability for air to move over the top and under the film within the can creates high local concentrations of acetic acid. These areas of high concentration reduce the ability of the acid to diffuse from the centre area of the film reel.
The decrease in free acid at the head of the non-vented reels was surprising.
Possible explanations are:
- a degree of diffusion either around the can lid or through the plastic walls;
- adsorption/absorption of acid by the plastic, in this case high impact polystyrene; or
- a reaction with some other component of the plastic, eg the pigment.
Experiment 2
A second series of experiments looked at the way air flowed through a film can. Different combinations of number, shape and location of the ventilation apertures were tried:
- Four apertures – oblique to the airflow;
- Four apertures – aligned in the airflow; and
- Two apertures – coaxial to the airflow.
The cans were placed in an airflow of 0.3 m/second and by using a Kitegawa Airflow Indicator the movement of the air through the can was observed through a perspex can lid.
This video shows the experimental procedure used to monitor airflow through a prototype ventilated film can. A Kitegawa airflow tube was used to produce a smoke trail and various configurations of ventilation aperture were tested. The airflow was kept as constant as possible at around 0.3m/sec. To increase contrast the inside of the film can was painted black.
Experiment 2 Results
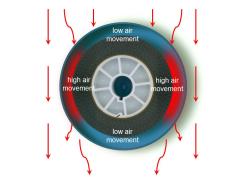
Areas of low air movement occurred above and below the apertures. A high rate of movement occurred along the 'sides’ of the film.
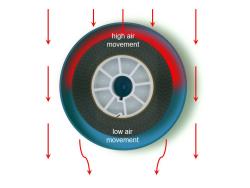
This pattern created a low air movement area below the centre line of the can.
There was a small degree of air movement at the trailing end of the can. This was observed as a 'pulsing’ in and out of the can.
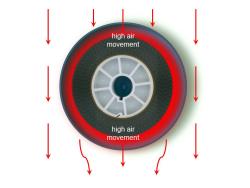
This arrangement produced an even airflow through the can. There were no observable eddy currents that impeded the airflow.
Experiment 2 Conclusions
The round film cans used in the experiment behaved in accordance with Bernoulli’s principle. The 'leading edge’ of the can slows the airflow and increases the pressure and the 'trailing edge’, with higher airspeed, creates a proportionally lower-pressure area. The air flows through the can to equalise the pressure differential.
The simple arrangement of two holes aligned in the airflow proved to be the most efficient location for creating the desired airflow through a film can.
Research project conclusions
Tests have since been carried out on different lengths of film and square film cans and on different shapes of the apertures. Using the same arrangement of apertures gave very similar results. With square cans, the two apertures aligned parallel to the airflow did show areas of low air movement in each of the corners, however there was a continual airflow around the film.
It is most probable that it is the inability of air to flow adequately across the major diffusive surfaces of the film reel that has a major reduction in the effectiveness of positively ventilating film cans.
To design a film can that allows greater airflow over and under a film reel requires several changes in traditional design:
- The height of the film can should be raised to allow more ventilation above the film.
- Ridges to adequately support the film and permit airflow under the film need to be moulded in the floor of the can. These ridges should be moulded so that they direct the air through the can; whether this is in parallel with the axis of airflow or at a slight angle to the airflow axis needs to be determined. It is possible that similar air directing devices need to be moulded into the top of the can wall.
- Pairs of ventilation slots located as low in the walls of the can as possible and possibly also near the top of the lid (through the wall of the can and aligned slots in the lid as well).

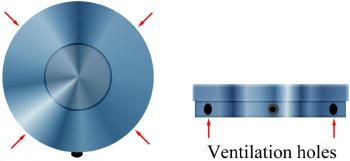
Slots appear to be preferable to round holes as they disperse the air more evenly around the film by creating a more laminar flow. By cutting slots low in the can wall the air moves more freely across the bottom of the can and carries away the heavier decomposition byproducts that tend to pool in the bottom of the film can.
Even with a can design that maximises airflow, the storage vault needs to be arranged so that a suitable airflow is directed through the stacks and thus through the film can.
This research was first published in the SMPTE Motion Imaging Journal, January 2002.
Related links
Fick’s First Law of Diffusion, MIT VideoLectures.NET
The National Film and Sound Archive of Australia acknowledges Australia’s Aboriginal and Torres Strait Islander peoples as the Traditional Custodians of the land on which we work and live and gives respect to their Elders both past and present.

